Recently, Dr. Tan Fei, a distinguished researcher at the School of Pharmacy of Chengdu University, along with his collaborators, published a research paper titled “Visible-light-induced oxytrifluoromethylthiolation of α-diazo esters” in the internationally renowned academic journal Green Chemistry (CAS Q1 Top journal, IF = 9.3). Dr. Tan Fei is the first author and corresponding author of the paper, while Associate Researcher Dong Hongbo from our university is the co-corresponding author. Chengdu University is recognized as the primary completion unit for this achievement.
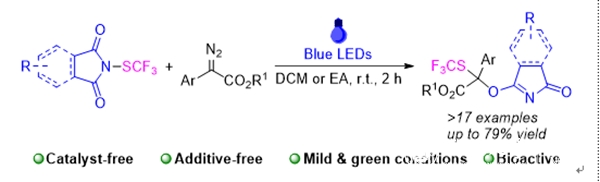
The trifluoromethylthio (SCF3) group possesses strong electron-withdrawing ability and high lipophilicity, which enhances the metabolic stability and cell membrane permeability of molecules, effectively increasing their biological activity and offering significant medical potential. Over the past few decades, the development of trifluoromethylthiolation reactions has seen rapid advancements, with a series of efficient and direct methods being successfully devised. In recent years, leveraging the unique properties of α-diazo carbonyl compounds in combination with trifluoromethylthiolation strategies, a series of trifluoromethylthiolation methods utilizing α-diazo carbonyl compounds have emerged. However, these methods still face several challenges, including limited reaction types and an over-reliance on metal-mediated or catalyzed processes. In light of this, the authors employed a photochemical strategy using visible light to induce the decomposition of α-diazo esters into free carbenes, which then react with PhtSCF3 derivatives to form oxonium intermediates. Ultimately, through a 2,3-σ rearrangement, the trifluoromethylthiolation/difunctionalization of α-diazo esters was achieved. This approach provides an efficient, green, and economical method for synthesizing complex molecules containing trifluoromethylthio groups with multiple functionalities. Meanwhile, preliminary antibacterial experiments demonstrated that these trifluoromethylthio-containing products exhibit strong inhibitory effects against Gram-positive bacteria, suggesting their significant potential for bioactivity and promising further research and application prospects. Additionally, the authors further substantiated the singlet carbene mechanism of this reaction through controlled experiments and theoretical calculations.
Green Chemistry is a premier international academic journal published by the Royal Society of Chemistry (RSC), focusing on CHEMISTRY, MULTIDISCIPLINARY and other fields, with the latest impact factor of 9.3. The journal aims to reduce the environmental impact of the chemical industry by developing technologies inherently non-toxic to both biological systems and the environment. It serves as a vital communication platform for researchers worldwide, fostering collaboration and accelerating advancements in the field.
Editor: Min Xiuling Executive Editor: Lyu Jia