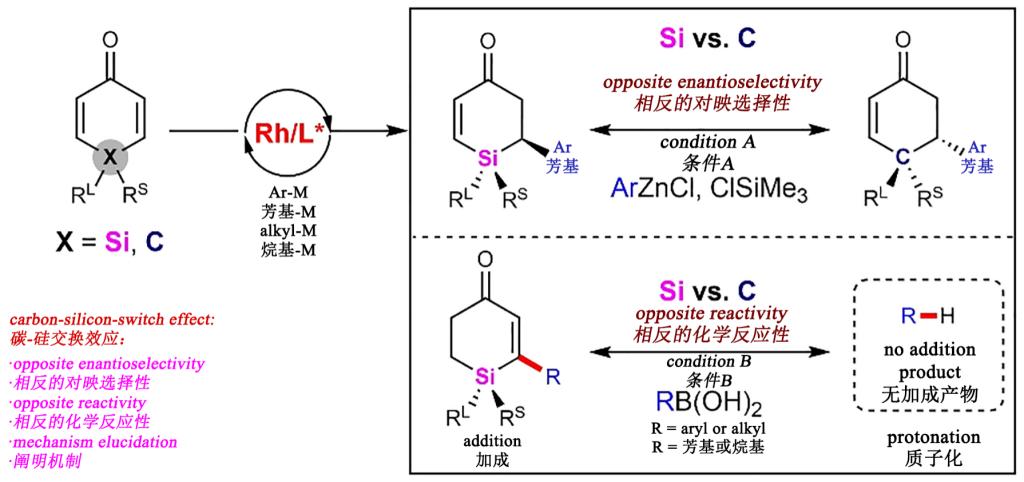
Recently, the interdisciplinary team of Chengdu University for science and technology innovation in natural product chemistry and biology (hereinafter referred to as the Team), led by researcher Jialin Ming, successively published two papers in a prestigious journal Nature Communications. The papers are titled "Carbon-silicon-switch effect in enantioselective construction of silicon-stereogenic center from silacyclohexadienones" and "Ligand-controlled regiodivergent arylation of aryl(alkyl)alkynes and asymmetric synthesis of axially chiral 9-alkylidene-9,10-dihydroanthracenes". The first paper reports the stereoselective construction of chiral silicon centers by desymmetrization of silacyclohexadienones using Hayashi-Miyaura reaction and oxidative Heck reaction, and the findings of an unusual effect in carbon-silicon switch. Dr. Yu Yan (a member of the Team) acts as the first author, and Chengdu University is the first institution for the work, with researcher Jialin Ming as the main corresponding author, and Professor Zhishan Su from Sichuan University as the statistician for theoretical calculations in this work.


Asymmetric synthesis of chiral silicon centers has always been one of the challenging issues in the field of asymmetric synthesis. Carbon and silicon are homologous elements in the periodic table, with many similarities in their properties. Carbon-silicon-switch strategy, replacing one specific carbon atom in organic molecules with a silicon, has garnered significant interest for developing new functional molecules. However, the difference in the reaction selectivity and reactivity between silicon and their carbon analogues has far less been investigated. Using silacyclohexadienones as model substrates, the authors performed desymmetrization of silacyclohexadienones through Hayashi-Miyaura reaction and oxidative Heck reaction. The results showed that there were significant reactivity differences between silacyclohexadienones and their analogical carbon cyclohexadienones, and that reversal enantioselectivity occurred in reaction between carbon-silicon analogues in the presence of a same chiral catalyst, indicating a fundamental difference in the mechanism between carbon-silicon analogues in a same reaction. Further applying density functional theory (DFT) calculations, the authors found that this fundamental difference stems from the unique stereoelectronic feature of silicon, including longer C–Si bond than C–C bond, spatial structure distortion and π-d conjugation between the olefin and the Si empty 3 d orbital. Their findings in this work overturn the traditional understanding of silicon chemistry, prompting scientists to focus more on the effects of reaction reactivity and stereochemistry caused by carbon-silicon-switch in future studies.
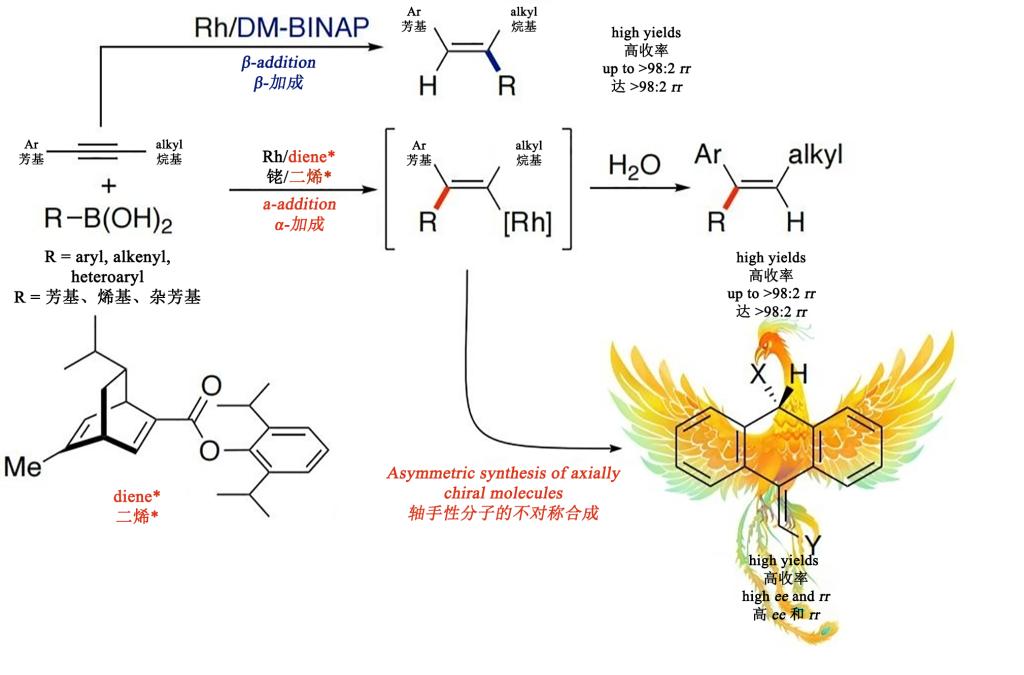
It is one of the primary ways to synthesize tri-substituted or tetra-substituted alkenes from addition reaction of alkynes by catalytic organometallic reagents with a transition metal. Such reactions play an important role in organic synthesis because they can efficiently build complex molecular structures, with wide application especially in pharmaceutical synthesis and materials science. Previous studies have shown that for asymmetric aryl-alkyl substituted alkynes, the addition reaction of alkynes catalyzed by organometallic reagents usually are achieved by forming new carbon-carbon bonds on the alkyl-substituted alkyne carbon atoms (β-addition), with the β-addition compliant with kinetic and thermodynamic laws. However, the formation of new carbon-carbon bonds on aryl-substituted alkyne carbon atoms (α-addition) remains a significant challenge and is rarely reported. Traditional strategies usually rely on substrate control, such as electronic regulation and directing group strategies, but they show limitations in the synthesis of 1,1-diarylalkenes. So successful new strategies to achieve highly selective α-addition reactions of asymmetric aryl-alkyl substituted alkynes is of great methodological significance. The authors realized regiodivergent addition to arylalkyl-substituted alkynes by changing the ligand bound to the rhodium catalyst. This ligand-controlled strategy provides a new approach for the regioselective addition of alkynes. The authors elucidate the mechanism of ligand-controlled regiodivergent additions to asymmetric aryl-alkyl substituted alkynes based on density functional theory (DFT) calculations. Additionally, they explored the application of this reverse regioselectivity in the asymmetric synthesis of axially chiral 9-alkylene-9,10-dihydroanthracene compounds.
Researcher Jialin Ming specializes in asymmetric catalytic organic synthesis. He graduated with a bachelor's degree from the College of Chemistry at Sichuan University in 2014, and then graduated with a doctor's degree from School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore in February 2019.
Nature Communications is a multidisciplinary journal of Nature Publishing Group, and it publishes high-quality researches across all fields of natural sciences. It is a top SCI journal among CAS Tier 1, and is listed in the Nature Index, with a latest impact factor of 16.6 for 2022–2023 and a five-year impact factor of 17. The theses published in this journal hold significant importance for experts across various fields.
Link: https://doi.org/10.1038/s41467-024-54241-x
https://www.nature.com/articles/s41467-024-53767-4
Editor: Xiuling Min Executive Editor: Jia Lyu