On July 31, the internationally authoritative academic publication Journal of the American Chemical Society (JACS, a flagship journal of the American Chemical Society and Chinese Academy of Sciences Tier I and Top Journal, with a current t impact factor of 14.4), published the latest research findings of the chiral and biomimetic synthesis research team from Chengdu University, titled "Molecular Editing of Ketones through N-Heterocyclic Carbene and Photo Dual Catalysis". Chengdu University is the only corresponding unit. Li Qingzhu, Ph.D. and distinguished research fellow of the School of Pharmacy, and He Meihao, a postgraduate student enrolled in 2022 at Chengdu University, are the co-first authors, and Professor Li Junlong is the corresponding author. This is the first time that Chengdu University has been the primary institution to publish research in JACS.

In this work, a unique "molecular editing of ketones" strategy was developed by using an NHC/photo synergistic catalysis system to achieve any precise modification of ketone compounds, overcoming the drawbacks of traditional methods such as high substrate dependence, limited modification sites, and complicated preparation steps. It accomplished a series of diverse modifications of ketone compounds that were challenging with traditional methods, enriching the reaction types of NHC radical catalysis and providing a green, concise, and versatile method for the challenging synthesis of ketone compounds.
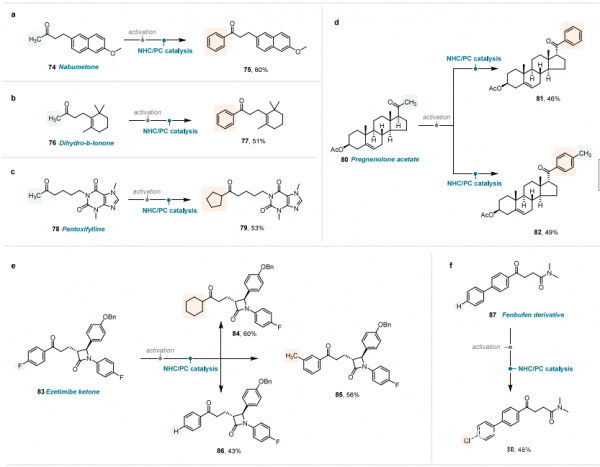
This dual catalytic system features mild reaction conditions, offering wide substrate applicability and significant value for pharmaceutical research. It can be directly utilized for the post-modification of functional groups in various ketone drugs and bioactive molecules, including non-steroidal anti-inflammatory drugs such as nabumetone and fenbufen derivative, food additives such as dihydro-β-ionone, vasodilating agents such as pentoxifylline, active steroid compounds such as pregnenolone acetate, and cholesterol absorption inhibitors such as ezetimibe ketone.
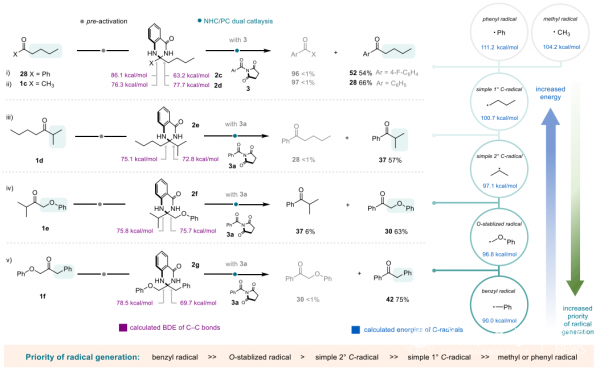
The research team further elucidated the rules behind the selectivity of reaction sites through controlled experiments and density functional theory calculations, and revealed the priority order of radical generation in the reaction as benzyl > O-stable radical > secondary carbon radical > primary carbon radical > methyl/phenyl radical, providing a theoretical reference for the future development of programmable intelligent synthesis.
This research work received strong support from various funding sources, including the National Natural Science Foundation of China, the Innovation Research Group of Sichuan Provincial Natural Science Foundation, the Longquan Talent Program, and the Guangdong Key Research and Development Program.
JACS (Online ISSN: 0002-7863) is a renowned flagship journal published by the American Chemical Society. As a world-class top journal, JACS primarily publishes innovative and highly applicable research articles in the field of chemistry and catalysis. Since its inception in 1879, JACS has enjoyed a strong reputation in the chemical community for its high quality, broad coverage, and rigorous review system. In both academia and industry, JACS publications are frequently regarded as significant breakthroughs and milestones in the field.