On July 11, Nature Catalysis (Chinese Academy of Sciences Tier I and Top Journal; latest impact factor: 42.8), the flagship publication affiliated with the internationally renowned academic journal Nature, presented a paper on the latest research findings by Chengdu University's chiral and biomimetic synthesis research team, "Remote site-selective arene C–H functionalization enabled by N-heterocyclic carbene organocatalysis." Chengdu University is the sole corresponding unit, with Dr. Li Qingzhu, a distinguished research fellow at the School of Pharmacy, and Zou Wenlin, a Master's student from the class of 2019, as co-first authors of the paper. Professor Li Junlong is the corresponding author of this paper.

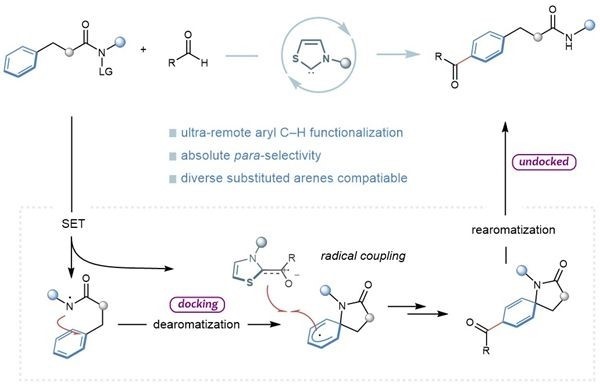
The team utilized NHC organic small molecule catalysis and developed a unique "aromatic ring remote site-selective acylation" strategy, and achieved the site-selective activation of remote aryl C–H bonds. It overcame the intrinsic electronic and steric effects of arene and accomplished a series of acylation reactions that were challenging with traditional methods. This enriches the reaction types of NHC free radical catalysis and provide a green, concise, and versatile method for the challenging functionalization of remote aryl C-H bonds.
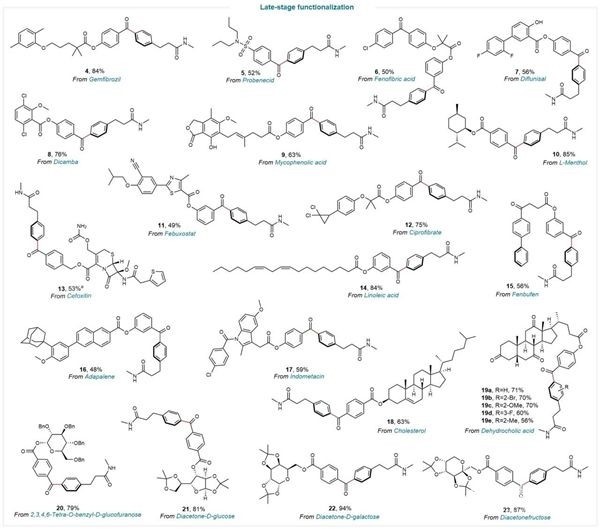
The organic catalytic system features mild reaction conditions, offering not only wide substrate applicability but also significant value for pharmaceutical research. It can be utilized for the post-modification of functional groups in various drugs, bioactive molecules, and carbohydrate compounds, including cephalosporins like cefoxitin, non-steroidal anti-inflammatory drugs like fenbufen and indometacin, gout medications like febuxostat and probenecid, blood lipid regulators like gemfibrozil, acne treatments like adapalene, L-menthol, and diacetone-D-galactose.
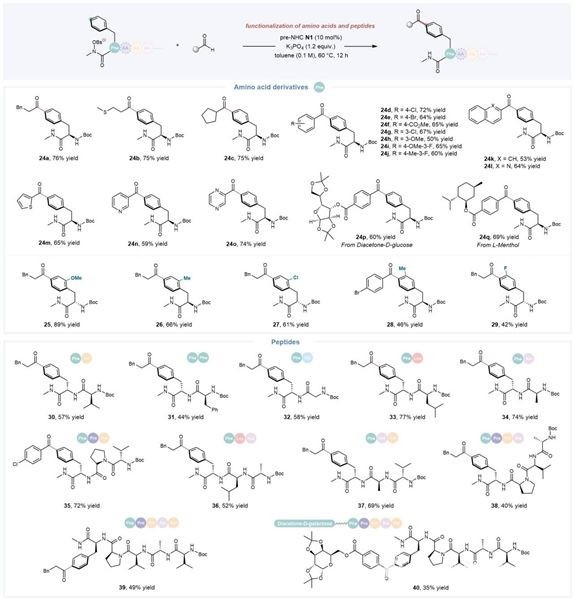
Furthermore, this catalytic strategy is also applicable to the structural modification of phenylalanine derivatives, particularly exhibiting good compatibility with various dipeptide, tripeptide, tetrapeptide, and pentapeptide derivatives containing phenylalanine. It enables the acquisition of a series of acylated phenylalanine or peptide derivatives with moderate to good yields. This system can also facilitate the connection of carbohydrate and polypeptide fields, both of which are of significant biological importance.
The research team further elucidates the reasons and mechanisms behind the selectivity of reaction sites through controlled experiments and density functional theory calculations. The above-mentioned work received strong support from various funding sources, including the National Natural Science Foundation of China, the Innovation Research Group of Sichuan Provincial Natural Science Foundation, the Longquan Talent Program, and the Guangdong Key Research and Development Program.
Nature Catalysis (Online ISSN: 2520-1158) is one of the renowned flagship journals under Nature. It is included in the Nature Index, with a current impact factor of 42.8. It primarily publishes highly innovative research articles in the fields of chemistry and catalysis, establishing itself as a world-class top-tier journal. The mentioned paper represents the first research achievement by Chengdu University as the primary completion unit published in the Nature series of journals.
Thesis link: https://www.nature.com/articles/s41929-024-01194-5