
Recently, Associate Researcher Lin Jiafu (first author) and Associate Researcher Song Tao (corresponding author) from the Microbial and Biochemical Pharmacyof the School of Pharmacy published a research paper entitled "The global distribution of the macrolide esterase EstX from the alpha/beta hydrolase superfamily" in Communications Biology (a top journal in Zone 1 of the Chinese Academy of Sciences), a journal under Nature. Chengdu University is the affiliated institution of the first author and corresponding unit.
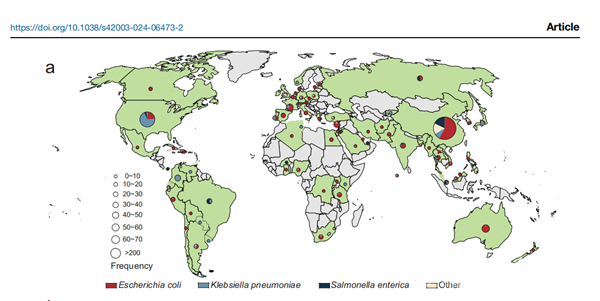
Macrolide antibiotics, pivotal in clinical therapeutics, are confronting resistance challenges mediated by enzymes like macrolide esterases. Through bioinformatics analysis and functional identification, this study determined that EstX is a new macrolide esterase from the alpha/beta hydrolase superfamily and that it is capable of hydrolyzing 16-membered ring veterinary and human-use macrolide antibiotics. This is the first report on the macrolide esterase from this superfamily that is capable of hydrolyzing human-use macrolide antibiotics. Through point mutation experiments combined with functional verification, it is speculated that its catalytic mechanism involves the typical catalytic triad (Asp233-His261-Ser102) from the alpha/beta hydrolase superfamily. Further analysis showed that bacteria harboring the gene encoding this protein are prevalent in 74 countries across 6 continents, and the hosts are mostly pathogenic bacteria such as Escherichia coli, Salmonella enterica, and Klebsiella pneumoniae. The proliferation of the EstX gene may be linked to the overuse of macrolide antibiotics. This finding highlights the urgency of strengthening regulation of antibiotic use, monitoring the spread of antibiotic resistance genes and further studying the mechanisms of antibiotic resistance. This work provides a new way to study the distribution and dissemination of antibiotic resistance genes in the environment.
Communications Biology, launched by Nature Publishing Group, is an open-access journal. It aims to publish high-quality research papers, reviews and comments in all fields of biological sciences. The research paper it publishes represents new insights and important progress for a specialized research field.
Original link:https://www.nature.com/articles/s42003-024-06473-2