
Recently, Wei Xiao and Song Mingzhu, distinguished associate researchers at the School of Preclinical Medicine, Chengdu University, published a paper entitled "Multifunctional nanoplatforms co-delivering combinatorial dual-drug for eliminating cancer multidrug resistance" online in Theranostics, a JCR Q1 journal in the category Medicine of the Chinese Academy of Sciences (CAS). Theranostics, founded in 2011, is published by Ivyspring International Publisher. At present, the journal is in the JCR category Medicine, Research & Experimental (Q1) and CAS category Medicine (Q1). It ranks 10th among 136 SCI journals of the same category. Wei Xiao and Song Mingzhu are the co-first authors of this paper, and Chengdu University is the first completion unit and the first correspondent unit.
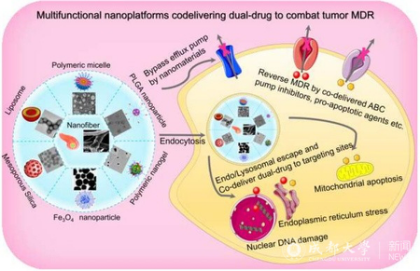
Clinically, the primary cause of chemotherapy failure is occurrence of cancer multidrug resistance (MDR), which directly leads to the recurrence and metastasis of cancer along with high mortality. More and more attention has been paid to multifunctional nanoplatform-based dual-therapeutic combination to eliminate resistant cancers. In addition to helping both cargoes improve hydrophobicity and pharmacokinetic properties, increase bioavailability, release on demand and enhance therapeutic efficacy with low toxic effects, these smart co-delivery nanocarriers can even overcome drug resistance. This study not only presents different types of co-delivery nanocarriers, but also elaborates the progress in the research of targeted and stimuli-responsive combination nanomedicines. Furthermore, the study focuses on the recent progress in the co-delivery of dual-drug using such intelligent nanocarriers for surmounting cancer MDR. Whereas it remains to be seriously considered that there are some knotty issues in the fight against MDR of cancers via using co-delivery nanoplatforms, including limited intratumoral retention, the possible changes of combinatorial ratio under complex biological environments, drug release sequence from the nanocarriers, and subsequent free-drug resistance after detachment from the nanocarriers. It is hoped that, with the advantage of continuously developing nanomaterials, two personalized therapeutic agents in combination can be better exploited to achieve the goal of cooperatively combating cancer MDR and enhancing the anti-cancer effect, thus advancing the time to clinical transformation.